UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other Jurisdiction
of Incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s
telephone number, including area code: (
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol(s) | Name
of Each Exchange on Which Registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
As previously announced on May 10, 2023, the management of Blue Water Biotech, Inc., a Delaware corporation (the “Company”), will present at the JMP Securities Life Sciences Conference at the New York Hilton Midtown on Monday, May 15, 2023 (the “JMP Conference”). The Company’s presentation, which was originally scheduled for 3:30 p.m. EDT, has been moved to 11:00 a.m. EDT. In addition, the form of the slide presentation the Company intends to use at the JMP Conference (the “Presentation”) is attached hereto as Exhibit 99.1 and is being furnished herewith.
The information in this Item 7.01 of this Current Report on Form 8-K (this “Current Report”) and the Presentation being furnished herewith shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01 and in the Presentation attached as Exhibit 99.1 to this Current Report shall not be incorporated by reference into any filing with the SEC made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| 99.1 | Presentation, dated May 2023. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
1
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Blue Water Biotech, Inc. | |
| Date: May 15, 2023 | /s/ Joseph Hernandez |
| Joseph Hernandez Chief Executive Officer |
2
Exhibit 99.1

` Corporate Overview & ENTADFI ® Opportunity May 2023 NASDAQ: BWV

Forward Looking Statements 2 The Presentation (the “Presentation”) has been prepared by Blue Water Biotech, Inc. (the "Company"). Certain information contained herein has been derived from sources prepared by third parties. While such information is believed to be reliable for the purposes used herein, the Company makes no representation or warranty with respect to the accuracy of such information. This Presentation does not constitute an offer to sell, or the solicitation of an offer to buy, any securities of the Company in any jurisdiction, domestic of foreign, where the offer, solicitation or sale is not permitted or would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. FORWARD LOOKING STATEMENTS: Certain statements in this presentation (the ”Presentation”) has been prepared by Blue Water Biotech, Inc . (the “Company”) . This presentation contains forward - looking within the meaning of the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others . These forward - looking statements are based on Blue Water current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, the Company’s ability to commercialize ENTADFI® ; market acceptance of the Company’s products and product candidates ; the size and growth of the potential markets for the Company’s products and product candidates and its ability to serve those markets ; the development of Blue Water’s vaccine candidates, including, but not limited to BWV - 301 ; the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations ; delays and uncertainties caused by the global COVID - 19 pandemic ; risks related to the timing and progress of clinical development of our product candidates ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and substantial competition . As with any vaccine under development, there are significant risks in the development, regulatory approval and commercialization of new products . Blue Water does not undertake an obligation to update or revise any forward - looking statement . Investors should read the risk factors set forth in Blue Water Annual Report on Form 10 - K for the fiscal year ended December 31 , 2022 , filed with the Securities and Exchange Commission (the “SEC”) on March 9 , 2023 and periodic reports filed with the SEC on or after the date thereof . All of Blue Water forward - looking statements are expressly qualified by all such risk factors and other cautionary statements . The information set forth herein speaks only as of the date thereof .

Executive Summary Experienced Management Team 1 • FDA - approved to treat BPH • BPH represents significant market opportunity • Official commercialization launch anticipated in Q3 2023 Substantial Opportunity with ENTADFI 2 Robust Vaccine Pipeline 3 3

Blue Water Biotech Overview Broad and Diverse Vaccine Pipeline Accomplished Management & Board of Directors Commercial Product Acquisition & Launch Focus on Diseases with High Unmet Need Vaccines against acute otitis media, pneumonia, influenza, norovirus, rotavirus, chlamydia and malaria Management team and board of directors with extensive and diverse industry experience Esteemed Research Collaborations University of Oxford, Cincinnati Children’s Hospital Medical Center, St. Jude Children’s Research Hospital, & UT Health San Antonio Recent purchase of ENTADFI ® highlights transition of Blue Water Biotech into a commercial company Targeting high - burden diseases & conditions that impact millions of lives globally Opportunistic Business Model & Corporate Strategy Exclusive licenses of assets & platforms and targeted business development efforts 4

Accomplished Management Team Led by experienced entrepreneurs with sustained records of successfully leading innovation and commercialization Joseph Hernandez Founder, Chairman & CEO Prior Management Experience Andrew Skibo Head of Biologic Operations Erin Henderson Chief Business Officer Ali Fattom, Ph.D. Head of Science and Discovery Jon Garfield Chief Financial Officer Frank Jaeger SVP of Marketing & Business Development Notable Products 5

Recent Vaccine Program Execution and Corporate Growth, Highlighted by Recent Purchase of ENTADFI ® Oct Dec Feb Mar Nov Jan Pneumonia Indication Expansion : Announced exploration of BWV - 201 efficacy in pneumococcal pneumonia Chlamydia Vaccine Development: Signed License Agreement with UT Health for Chlamydia Vaccine Development Key Opinion Leader Event: Hosted KOL event to discuss BWV - 201 approach & development timeline Institutional Research Coverage: “Buy” rating obtained from H.C. Wainwright ($4 PT) and Maxim Group ($7 PT) AbVacc Collaboration: Signed co - development agreement with AbVacc for joint development of monkeypox & Marburg vaccine candidates Frank Jaeger Appointment: Appointed Frank Jaeger as Senior VP of Marketing and Business Development and lead for ENTADFI launch Apr May ENTADFI ® Acquisition & Corporate Name Change: Acquired commercial asset ENTADFI and subsequent corporate name change to Blue Water Biotech Blue Water anticipates commercial launch of ENTADFI ® in Q3 2023 6

ENTADFI ® A Transformative Opportunity 7

Total US BPH Target Population of 55.1 Million Men 50+ Years of Age 1,3 Benign Prostatic Hyperplasia (BPH) Overview 8 Delay in symptom relief and sexual adverse effects lead to poor adherence of current BPH treatments Benign Prostatic Hyperplasia – The Disease • Histologic prevalence globally is thought to be ask high as 50% for men in their 50s, 70% for men in their 60s, and 80% for men 70 years of age or older 1 • Medical management is most common treatment option for symptomatic disease • Common BPH symptoms include frequency, urgency, and an inability to void • Up to 70% of BPH patients have erectile dysfunction 2 • Per IQVIA, 44M US BPH prescriptions were filled in 2022 • Prescriptions are written by both primary care and urology BPH – The Need • No current BPH prescriptions provide symptom relief, prostate size reduction, and ED treatment • ENTADFI® will be the first line prescription to treat BPH symptoms, reduce prostate size, and manage ED symptoms

9 LUTS/BPH severity and number of medications influence adherence rates • Men with less severe symptoms have poorer adherence 3 • Men taking multiple BPH treatments concurrently had an adherence rate of 9% 4 Men with moderate - to - severe LUTS are at increased risk for sexual dysfunction , including erectile dysfunction, ejaculatory dysfunction, and hypoactive desire 2 Several BPH treatments significantly increased the risk of ED , ejaculatory dysfunction, and hypoactive sexual desire in subjects with BPH 3,4 AEs related to sexual/ejaculatory dysfunction appear to increase with 5 - ARI/ α - blocker coadministration 1 Current BPH treatment can take up to 6 - 12 months for significant symptom relief , contributing to adherence concerns 1 Lower urinary tract symptom improvement is not observed with finasteride monotherapy for 6 to 12 months and α - blockers are not indicated to reduce prostate size 1 5 - ARI – 5 - alpha reductase inhibitor; AE – adverse events; BPH – benign prostatic hyperplasia; ED – erectile dysfunction; LUTS – lower urinary tract symptoms. References: 1. Casabé A et al. Journal of Urology . 191:727 - 733 2014. 2. Rosen RC, et al. European Urology . 2005;47(6):824 - 837. 3. Zabkowski T, Saracyn M. J Physiol Pharmacol . 2018;69(4):10.26402/jpp.2018.4.14. 4. Cindolo L, et al. European Urology . 2015;68(3):418 - 425. 5. Shin YS, et al. World Journal of Men’s Health . 2019;37(2):157 - 165. 6 . Corona G, et al. Andrology . 2017;5(4):671 - 678. The ENTADFI Opportunity ENTADFI has the potential to be first - line tx for BPH symptoms, reducing prostate size and managing ED

ENTADFI ® is the First and Only FDA - Approved Combination Therapy for BPH Tadalafil Finasteride Dual MOA Faster & improved LUTS Greater relief of LUTS Sustained over 26 weeks Key Differentiators Significant 1 Σ & 2 Σ endpoints (at all time points) > Treatment satisfaction at week 26 10

- 4.0 - 5.2 - 5.5 - 2.3 - 3.8 - 6.0 - 5.0 - 4.0 - 3.0 - 2.0 - 1.7 - 1.4 - 4.5 - 1.0 Tadalafil / Finasteride Placebo / Finasteride WEEK 0 WEEK 4 WEEK 12 WEEK 26 LS mean change from baseline Primary Endpoint Achieved • LS mean change from baseline with TAD/FIN at 12 weeks was - 5.2 vs - 3.8 for PBO/FIN (LSTD of - 1.4 [95% CI - 2.3, - 0.6; p ≤0.001]) Key Secondary Endpoints Were Statistically Significant • Significant LUTS improvements were observed with TAD/FIN at 4 and 26 weeks after baseline • LS mean change in I - PSS total score – Week 4: TAD/FIN was - 4.0 vs - 2.3 for PBO/FIN (p <0.001) – Week 26: TAD/FIN was - 5.5 vs - 4.5 for PBO/FIN (p = 0.022) International Prostate Symptom Score (IPSS): an eight - question self - administered survey used to screen for, diagnose, track, and manage the symptoms of BPH. Higher scores correlate with more severe symptoms and decreased QoL Time course of LS mean change from baseline in I - PSS total score I - PSS Change from Baseline 0.0 0.0 - 1.0 TAD/FIN Led to a 74% Greater Reduction than Finasteride Alone Within the First 4 Weeks and a 22% Greater Reduction at Week 26 11 ENTADFI ® Significantly Improves Early BPH Symptoms

12 BPH – benign prostatic hyperplasia; ED – erectile dysfunction; FIN – finasteride; HCP – healthcare provider; IIEF – International Index of Erectile Function; LS – least square;; PBO – placebo; TAD – tadalafil. 0 .0 3.7 4.7 4.7 - 1.1 0.6 0.0 - 2.0 - 1.0 0.0 1.0 2.0 3.0 4.0 5.0 IIEF - EF Change from Baseline Men With Baseline ED Tadalafil / Finasteride Placebo / Finasteride WEEK 0 WEEK 4 WEEK 12 WEEK 26 0 .0 0.8 3.1 2.5 - 1.9 0.0 - 1.0 - 3.0 - 2.0 - 1.0 0.0 1.0 2.0 3.0 4.0 IIEF - EF Change from Baseline Men Without Baseline ED Tadalafil / Finasteride Placebo / Finasteride WEEK 0 WEEK 4 WEEK 12 WEEK 26 Among Sexually Active Men With and Without Baseline ED Treated with TAD/FIN, Significant Improvements Were Observed in Scores at All Three Postbaseline Timepoints that were Significantly Greater than Patients Treated with PBO/FIN 1,2 References: 1. Casabé A et al. Journal of Urology . 191:727 - 733 2014. 2. Glina et al. J Sex Med . 2015;12:129 – 138. The International Index of Erectile Function (IIEF): a widely - used multidimensional evaluation for male sexual function. A self - administered questionnaire that reliably assesses sexual function & satisfaction to help inform HCPs of sexual symptoms associated with BPH ENTADFI ® Significantly Improves Sexual Function in Men

Maximizing ENTADFI ® Potential 13

At Close to 44 MM TRx Written Per Year, the BPH Market is Large and Growing 14

15 Source: 1. Feinstein L., Matlaga B. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and 23% of all urologic office visits BPH treatment and diagnosis make up the largest segment of urologic practice 2 54.8% of 12.2M actively managed BPH patients are managed with pharmacological therapy 2 41.2% of privately insured BPH patients 41.2% of privately insured BPH patients filled at least one BPH - related prescription 1 12.2M Approximate Medicare annual in - office and outpatient BPH service costs 1 $1.5 BILLION Kidney Diseases; 2018; NIH Publication No. 12 – 7865 [pp. 78 – 81]. 2. Vuichoud, C. Canadian Journal of Urology 22.Suppl 1 (2015): 1 - 6. Medicare was estimated to have spent more than $1.5 billion on in - office and outpatient services related to LUTS - BPH 1 actively managed with BPH treatment Treatment of Lower Urinary Tract Symptoms Still Remains Problematic in the United States

Market Research ENTADFI’s product profile highly accepted by Urologists indicating quick adoption 16

HCP Education 17 Consumer Education Partnerships Three - Pronged Strategy to Commercialize ENTADFI ® and Transform Blue Water Biotech

Potential Men’s Health Strategic Partners 18

Direct to Consumer Reaching diagnosed and treated patients to educate on ENTADFI Patient awareness Direct - to - consumer advertising Patient demand 19
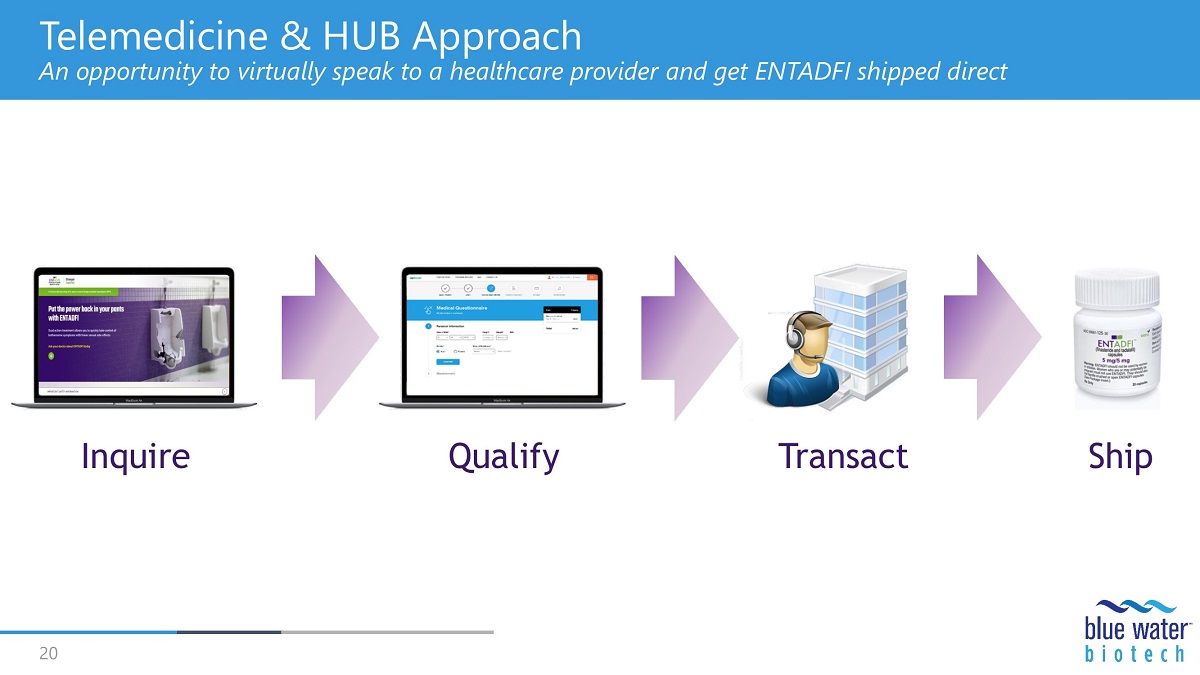
Telemedicine & HUB Approach An opportunity to virtually speak to a healthcare provider and get ENTADFI shipped direct Inquire 20 Qualify Transact Ship

Healthcare Provider Focus Hyper - focused Targeting directed to top decile Urologists Decile Total Rx’s Total URO Rx per HCP 10 1,046,034 229 4,568 9 1,044,673 327 3,195 8 1,044,958 402 2,599 7 1,043,744 470 2,221 6 1,049,786 553 1,898 5 1,045,625 648 1,614 4 1,040,716 766 1,359 3 1,043,213 960 1,087 2 1,043,741 1,334 782 1 1,043,245 7,069 148 21 ~2,600 HCP’s fall into Urology Deciles 5 - 10 * 2023 Deciling, IQVia LRx claims

Intellectual Property Unique formulation and manufacturing know - how creates barrier to entry by generic competition 22

Our Vaccine Candidates 23

Broad and Diverse Vaccines Pipeline Infectious Disease Program Candidate Preclinical Phase 1 Phase 2 Phase 3 Collaborator S. pneumo - Induced Acute Otitis Media & Pneumonia BWV - 201 Universal Flu BWV - 101 H1 Pre - Pandemic BWV - 102 Norovirus / Rotavirus BWV - 301 Malaria BWV - 302 Monkeypox AbVacc Collaboration Marburg AbVacc Collaboration Chlamydia BWV - 401 24

Renowned Research Partners Sunetra Gupta, Ph.D. Co - Inventor, Universal Influenza Vaccine (BWV - 101) Professor, University of Oxford Xi Jason Jiang, Ph.D. Co - Inventor, S & P Particle VLP Platform, Norovirus - Rotavirus Vaccine (BWV - 301) Retired Professor, University of Cincinnati, Department of Pediatrics Ming Tan, Ph.D. Co - Inventor, S & P Particle VLP Platform, Norovirus - Rotavirus Vaccine (BWV - 301) Assistant Professor, University of Cincinnati, Department of Pediatrics Jason Rosch, Ph.D. Inventor, S. pneumoniae Vaccine (BWV - 201) Associate Member, St. Jude Faculty Guangming Zhong, M.D., Ph.D. Inventor, Chlamydia Vaccine (BWV - 401) Professor, University of Texas Health San Antonio 25

26 Targeted Vaccine Pipeline to Address High Disease Burden and Areas Without Efficacious Vaccines BWV Program Target Indication Market Size Current Development Phase BWV - 201 AOM & Pneumonia AOM : $4B spent on treatment annually 1 Pneumonia : $1.3B in direct medical costs in the US annually 2 Preclinical, cGMP Manufacturing - Ready BWV - 101 Universal Flu Total annual economic burden due to influenza is approximately $87B in the US alone 3 Preclinical, Epitope Optimization BWV - 102 H1 Pre - Pandemic Limited ability to develop pre - pandemic vaccines given yearly reformations of vaccines & lack of broad vaccine coverage Preclinical, Epitope Optimization BWV - 301 Norovirus / Rotavirus Norovirus: 700 million cases 4 & $60.3B spent worldwide annually 5 Rotavirus: 111 million cases each year & limited vaccine efficacy in LIC 6 Preclinical, VLP Expression BWV - 302 Malaria $12B in direct medical costs worldwide each year 7 , limited vaccine available vaccines 8 Preclinical, VLP Optimization BWV - 401 Chlamydia 1.6 million cases in the US each year 9 , 129 million globally 10 , no current vaccines available 11 NHP Study in 2023 1) 2) 3) 4) 5) 10.1016/j.vaccine.2007.03.046. Epub 2007 Apr 20. PMID: 17544181. Centers for Disease Control and Prevention, “Norovirus Worldwide” Tan M. Norovirus Vaccines: Current Clinical Development and Challenges. Pathogens. 2021 Dec 19;10(12):1641. doi: 10.3390/pathogens10121641. PMID: 34959596; PMCID: PMC8709042. Tong S, Amand C, Kieffer A, Kyaw MH. Trends in healthcare utilization and costs associated with acute otitis media in the United States during 2008 - 2014. BMC Health Serv Res. 2018 May 2;18(1):318. doi: 10.1186/s12913 - 018 - 3139 - 1. PMID: 6) 29720156; PMCID: PMC5932897. 7) CDC: Drug Resistant Streptococcus Pneumoniae. https:// www.cdc.gov/drugresistance/pdf/threats - report/strep - pneumoniae - 508.pdf 8) Molinari NA, Ortega - Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007 Jun 28;25(27):5086 - 96. doi: 9) World Health Organization, “Rotavirus Vaccines: WHO position paper – July 2021” Centers for Disease Control and Prevention, “Malaria’s Impact Worldwide” World Health Organization, “WHO recommends groundbreaking malaria vaccine for children at risk”, 6 October 2021 Centers for Disease Control and Prevention, “The State of STDs in the United States in 2021” World Health Organization, Fact Sheets, “Sexually Transmitted Infections (STIs)” Centers for Disease Control and Prevention, “Chlamydia Treatment & Care” 10) 11)

27 Executive Summary Experienced Management Team 1 • FDA - approved to treat BPH • BPH represents significant market opportunity • Official commercialization launch anticipated in Q3 2023 Substantial Opportunity with ENTADFI 2 Robust Vaccine Pipeline 3

` Thank you! Follow us on: https://www.facebook.com/BlueWaterVaccines https://www.linkedin.com/company/blue - water - biotech - inc https://twitter.com/BlueWater_Bio
